
A Waring blender, similar to the one used by Hershey and Chase.
It started with a blender. A Waring blender, to be exact. The thing is, until a retro green model caught my eye in a kitchen store, I’d never seen one. I’d heard the name years before in a high school classroom in the context of bacteria, protein, and DNA. It was the brand used in the Hershey-Chase blender experiment that convinced scientists in the early 50’s that DNA, not protein, was the molecule of heredity. As a high schooler, I had no clue what a real-life research lab was like, and when I heard “Waring blender” in a breathless phrase, I pictured something industrial-grade, special. It didn’t occur to me that in 1952, while Alfred Hershey and Martha Chase agitated bacteria, people were making Mai Tais with the same appliance.
As a graduate student at Cold Spring Harbor Laboratory (CSHL), the very place Hershey and Chase performed the blender experiment, I’ve come to know that scientists are a scrappy, resourceful bunch. We use what works, and the mundane blender at the center of the famous experiment did exactly what Hershey hoped it would.
Back in 1952, “genes” weren’t synonymous with DNA. Genetic information was thought of as the material that enabled an organism to reproduce, but it was still controversial as to what that material was. Experiments by Avery, MacLeod, and McCarty at Rockefeller University in 1944 provided evidence that this material was DNA, but they faced considerable resistance from the scientific community. Hershey himself favored the theory that proteins, with their more complex forms, harbored genetic information. When Hershey and Chase set out to conduct an experiment that could settle this issue once and for all, Hershey probably had in mind disproving the Avery-MacLeod-McCarty result. However, the blender experiment results proved to be a “mental shock” that rocked Hershey’s views. Once the careful experimentalist convinced himself that DNA was the molecule of heredity, he convinced the world.

Electron micrograph taken by Anderson shows the T4 phage infecting an E. coli bacterium. The infecting virus stays attached by its tail the surface of the bacterium, as new virus is being produced inside the cell. Shown with diagram of T2 phage, with protein coat and DNA in the head. (T2 diagram, Wikimedia Commons)
The simple viral-host system of T2 bacteriophage and E. coli was the perfect battleground on which to pit the protein theory of heredity against its DNA competitor. The reason being that T2 phage is made up of only two parts: a protein coat and the DNA contained therein.
Just months before the blender experiment, electron micrographs taken by Thomas Anderson showed that phage never invades bacteria as a whole: it always remains attached at the surface. Some part of the phage – either its DNA or certain proteins – must enter the bacterium in order to hijack its cellular machinery and produce new virus.
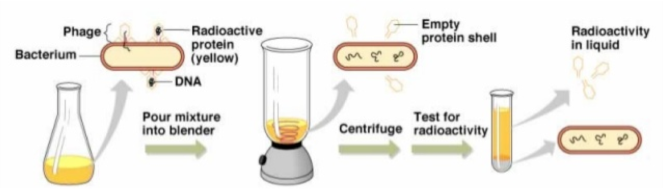
In an experiment many biologists would gladly give their pipetting arm to have designed, Hershey and Chase tracked the location of T2 phage DNA during its hostile takeover of E. coli by tagging it with a radioactive form of phosphorous. DNA contains vastly greater amounts of phosphorous compared to protein, so the presence of high levels of radiation would give away phage DNA’s location. Hershey and Chase wanted the tagged phage to infect bacteria, but they didn’t want it to stick around at the surface – they only cared about what the phage transferred to the bacterium during the initial stage of infection.
This is where the Waring blender got its chance to shine.
The blender wasn’t their first choice for removing phage from the outside of bacteria. According to Hershey, “We tried various grinding arrangements, with results that weren’t very encouraging”. It was only after a fellow CSHL geneticist, Margaret McDonald, offered her blender that the experiments started to work. The Waring blender had just the right amount of shearing force to remove phage coats from the bacterial walls, without rupturing the bacteria completely. They then spun the bacteria/phage smoothie in a centrifuge to separate infected bacteria from everything else: bacteria formed a pellet at the bottom of the tube, while everything that was smaller (including protein coats) stayed in the liquid above it. Upon measuring radioactive phosphorous, Hershey and Chase found that only bacteria showed high levels of radiation. DNA made it into the bacteria, protein didn’t. Most importantly, phage were able to replicate in bacteria even when their protein coats were kicked off soon after infection.

It looked like protein itself wasn’t necessary for phage reproduction after all. It might just serve as a passive vehicle to get the genetic material into the cell. At first, Hershey thought there must be a mistake. After presenting the blender experiment results in a staff meeting, a young scientist named Waclaw Szybalski recalled Hershey said, “I don’t believe in that DNA”. Meanwhile, Barbara McClintock thought it was a nice experiment.
Hershey’s skepticism is a testament to his scientific rigor, considering that he stared at a near perfect explanation for his results around this time. In a letter to Hershey, Roger Herriott wrote,
I’ve been thinking – and perhaps you have been too – that the virus may act like a little hypodermic needle full of transforming principles; that only the tail [of the virus] contacts the host and perhaps [sic] cuts a small hole through the outer membrane and then the nucleic acid of the virus head flows into the cell.
Hershey and Chase repeated their experiment, only this time they tagged the phage protein by radiolabeling an element found only in protein: sulfur. They found the exact opposite result: radiation was found in the liquid portion that contained the protein coats and not in the bacteria. Again, the virus was able to replicate in the bacteria cells after blending had removed protein coats from the cell walls. Further experiments showed that phage offspring contain radioactive phosphorous passed down from the tagged parental phage. Virtually no radioactive phosphorous is found in the protein of viral replicates. This finding further solidified the notion that DNA was the genetic material responsible for reproduction.
A mere 11 months after reading a letter from Hershey detailing the results, James Watson along with Francis Crick solved DNA’s structure, unlocking its hidden code. The Hershey-Chase experiment convinced Watson that the 3D structure of DNA was worth chasing, and to say that DNA was the next big thing in biology would be an understatement. Still, Hershey’s cautious personality shone through the last sentence of his and Chase’s groundbreaking paper, “Further chemical inferences should not be drawn from the experiments presented.”
*******
This post is a result of a CSHL tour I gave to a high school class this week. The teacher asked me in which building Hershey and Chase performed their blender experiment. I took a safe guess at McClintock Laboratory, which back in the day was the animal house. While correct, it made me want to refresh my memory of the experiment. As a working scientist, I have a much greater appreciation for its simplicity and elegance than I could at 15.

Martha Chase and Alfred Hershey at CSHL, 1953
Just as I naively took “Waring blender” to be a single entity, I saw an equal scientific partnership in the symmetry of the “Hershey-Chase” experiment’s name. Instead, Martha Chase was Hershey’s young lab technician. Hershey wrote that the two were “groping toward the idea of the blender experiment” in 1951, implying that she did aid in the experiment’s design. However, I have yet to find any account of the work in Martha’s own words. While Hershey went on to win the Nobel Prize in 1969 with other Phage Group pioneers, Max Delbruck and Salvador Luria, Martha’s life took a more tragic trajectory.
In my reading I came across a post on a blog about the scientist Linus Pauling called “The Martha Chase Effect.” The author found that while their blog was dedicated to the life and work of Linus Pauling, the search term that most frequently brought people to the site was, “Martha Chase”. This is because Chase’s digital footprint, particularly for images, “represents a much smaller body of water” than Pauling’s.
A puddle, really.
In a future post, I hope to explore the “Martha Chase Effect” and learn more about the young woman whose name is forever eponymous with the Waring blender experiment.
Edit:
Take a gander at the actual blender used in the experiment: not as sleek as the one I showed (notice the warning label for radioactive P32).

Thanks to Matthew Cobb for the link!
Sources:
Thanks to The Linus Pauling blog, for background information and inspiration for the Martha Chase Effect.
“A Phage Shows its Claws” by Lee Simon in New Scientist and Science Journal, 1971.
“The Injection of DNA into Cells by Phage” by A.D. Hershey from Phage and the Origin of Molecular Biology CSHL Press.
“Martha Chase Dies” by Milly Dawson in The Scientist Aug 20, 2003.
“Independent Functions of Viral Protein and Nucleic Acid in Growth of Bacteriophage” by Hershey and Chase in The Journal of General Physiology. 1952
CSHL Oral Histories: Waclaw Szybalski on Martha Chase.





















 Once a scientist unlearns his or her bad habits, the next step is to practice the craft of story building. Zimmer said that when we look at a beautiful piece of architecture, what we appreciate is how the craftsmanship at different scales work together. In writing, the craft starts with word selection and scales up to the paragraphs and overall structure of the story. Like a building, we can take apart the elements of a story and figure out how it was constructed and what makes it compelling. If you want to learn how to write a magazine article, reverse engineer a magazine article.
Once a scientist unlearns his or her bad habits, the next step is to practice the craft of story building. Zimmer said that when we look at a beautiful piece of architecture, what we appreciate is how the craftsmanship at different scales work together. In writing, the craft starts with word selection and scales up to the paragraphs and overall structure of the story. Like a building, we can take apart the elements of a story and figure out how it was constructed and what makes it compelling. If you want to learn how to write a magazine article, reverse engineer a magazine article.



 Neuroscientists at Stanford have discovered a brain pathway that controls a rat’s will to fight in a challenging situation.
Neuroscientists at Stanford have discovered a brain pathway that controls a rat’s will to fight in a challenging situation. Scientists in
Scientists in 


